Case Study Riverside Health System Drives Operational Efficiencies and Process Improvement
See how this 450-bed regional hospital decreased ED TATs by 26%, decreased errors by 40% and increased labor savings by 10%.
DownloadExecutive Summary
Profile
Riverside Health System is a network of five individual hospitals. The core lab, Riverside Regional Medical Center in Newport News, Virginia, performs 3.5 million tests annually for its 450-bed facility and operates 24/7.
Challenge:
Whether it was labor-intensive management of varied instrumentation, a lack of standardization in processes and decision rules or pending staff reductions due to retirements, challenges amounted for the growing network. Increasing test volumes, largely driven by outpatient business and heightened emphasis on specimen tracking and patient safety, added to the concerns.
Solution:
- Power Processor Sample Handling System
- Two UniCel DxC 800 clinical chemistry analyzers
- Two UniCel DxI 800 immunoassay analyzers
- 3,000-tube stockyard
- REMISOL Advance Middleware
|
|
Transformation
As the core laboratory for a network of five hospitals, Riverside Regional Medical Center was faced with the challenges associated with delivering fast, accurate results and consistent care for both ED and outpatient services. The laboratory’s goals were to address increasing testing volumes, compensate for staff shortages, decrease TATs while reducing the number of outliers, reduce costs and add revenue.
To meet these goals, the Riverside Regional Health System selected Beckman Coulter.
Phase 1: Implementing instrumentation, automation and REMISOL Advance Middleware
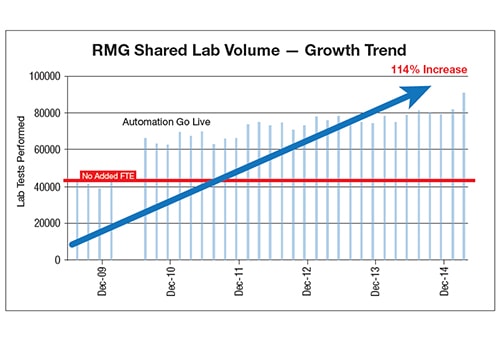
The core lab and two sister hospitals within the health system upgraded chemistry, immunoassay and automation instrumentation. Knowing automation would help the laboratory meet its need for productivity and coverage, Riverside Regional Health System helped to justify costs by consolidating operations with a local free-standing outpatient laboratory.
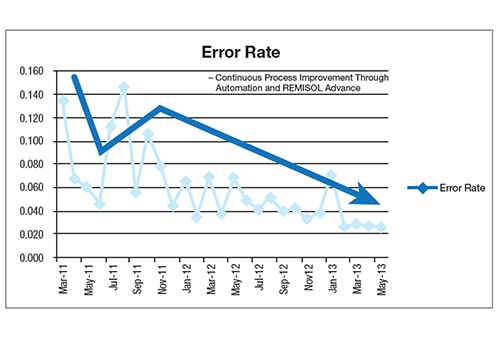
After implementation, the hospital saw continuous process improvement through automation, REMISOL Advance Middleware with EQC and EMWA. EWMA, in particular, virtually eliminated errors related to instrument shifts, enabling the laboratory to catch issues in real time, saving it from releasing erroneous results. REMISOL Advance Middleware was eventually deployed at other sites to offer a safety net for stopping erroneous results reporting and enabling sample traceability.
Phase 2: Adding autoverification
The laboratory was able to overcome previous challenges in implementing autoverification by using IT support and challenges which simplified rule building and reduced the amount of IT support needed. However, with increasing test volumes, ongoing manual verification was not sustainable. Adopting autoverification, in combination with automation, helped the hospital keep up with a workload that doubled in three years. The hospital also saw decreased TAT and reduced TAT ranges.
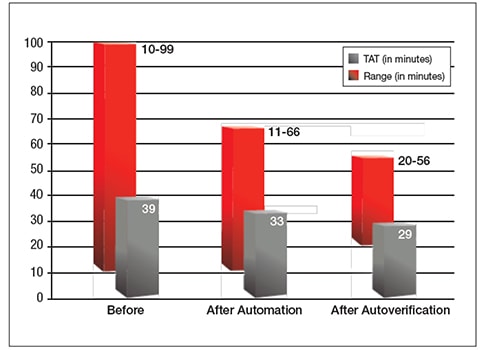
Additionally, after autoverification, there was a 10% decrease in staff overtime in the first month, a reallocation of resources to save another one-half of an FTE and a cost savings of $8,000–$10,000 previously spent on paper and toner cartridges.
Power Processor Sample Handling System
UniCel DxC 800 clinical chemistry analyzer
UniCel DxI 800 immunoassay analyzer
REMISOL Advance Middleware
REMISOL Advance is a trademark or registered trademark of Normand-Info SAS in the United States and other countries. Used under license. †Not available in all regions. Contact your local Beckman Coulter representative for availability.
Never miss another insight
Subscribe to our Diagnostics Today newsletter to stay up to date on the latest news in clinical diagnostics.
 English
English


