| Assays | Education | Request more |
Rising to a Global Challenge
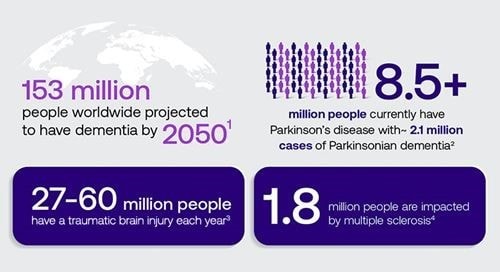
Fueled by epidemiological factors including population growth and longer life expectancies, cases of neurodegenerative diseases are expected to triple by 2050,1 placing financial and emotional burdens on patients, their caregivers, and healthcare systems. Current neurodegenerative disease diagnostics are limited by non-specific clinical symptoms, challenges in early diagnosis, and the high cost of available testing, creating an urgent need for affordable, scalable, and accessible blood-based diagnostics.
Leveraging our decades of IVD expertise, we’re creating best-in-class, highly sensitive, easy-to-use assays to ultimately improve testing accessibility and enhance clinical decision making to forever change how neurodegenerative diseases are investigated, diagnosed, and monitored
 English
English