What is covered under IVDR?
Any in vitro diagnostic medical device that is a reagent, reagent product, calibrator, control material, kit, instrument, apparatus, piece of equipment, software or accessory, whether used alone or in combination, intended by the manufacturer to be used in vitro for the examination of specimens, including blood and tissue donations, derived from the human body, solely or principally to provide information on one or more of the following:
- Concerning a physiological or pathological process or state
- Concerning congenital physical or mental impairments
- Concerning the predisposition to a medical condition or a disease
- To determine the safety and compatibility with potential recipients
- To predict treatment response or reactions
- To define or monitoring therapeutic measures
Specimen receptacles shall also be deemed to be in vitro diagnostic medical devices.
What is the difference between IVDR and IVDD?
Some of the main differences between the new In Vitro Diagnostic Regulation and the previous In Vitro Diagnostics Directive are: 1,2
- Scope and classification of products
- Improved transparency and data for the European Database for Medical Devices (EUDAMED), the European Union’s database for medical devices
- Role of Economic Operators (clarification of obligations of manufacturers, authorized representatives, importers and distributors)
- Changes to notified bodies
- Addition of Unique Device Identification (UDI) System for every IVD device
- Changes to clinical evaluation processes with an increase in clinical evidence and conformity assessment
Device risk-classification categories
-

Risk level: Low
Review process:
Manufacturer self-declaration
Examples:
General lab instruments, specimen receptacles
-

Risk level: Low-Moderate
Review process:
Notified body review required
Examples:
Some self-test products such as pregnancy, fertility, cholesterol and devices that detect glucose
-

Risk level: Moderate-High
Review process:
Notified body review required
Examples:
Cancer tests, genetic tests, congenital screening of embryos, fetuses or new-born babies
-

Risk Level: High
Review process:
Notified body review required
Examples:
High risk diseases, blood screening of blood components, determining the infectious load of a life-threatening disease
What is the IVDR implementation timeline?
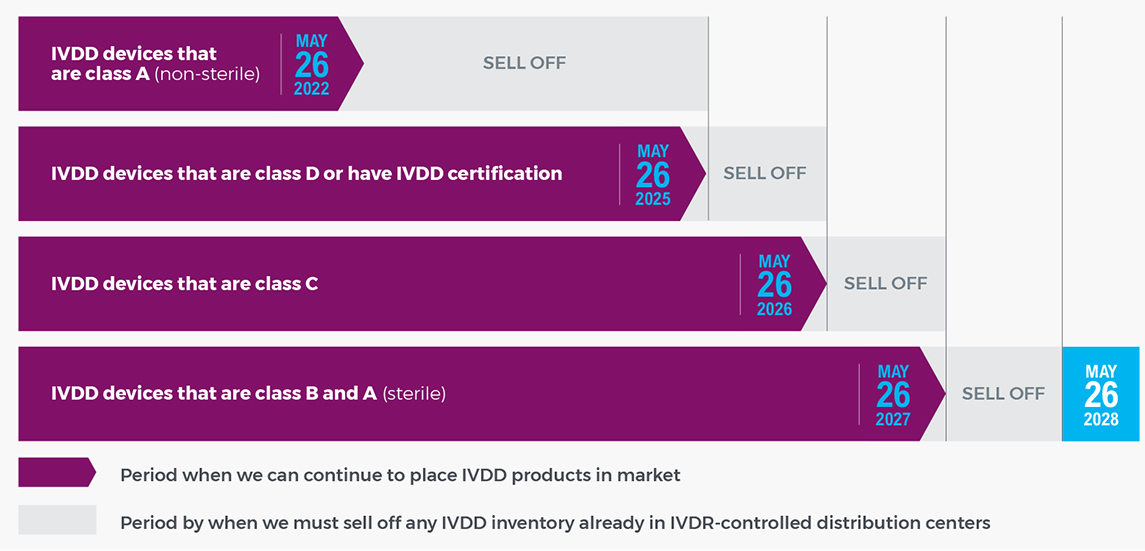
How does this impact me as a customer?
Beckman Coulter is working to ensure all relevant products are IVDR-compliant and have processes in place for recertification to ensure product continuity for all our customers.4
IFU Changes
IFUs will be updated to include the CE Notified Body Number alongside the CE mark.
Other changes to IFUs may include:
- Intended purpose statements
- Warnings or contraindications
- Additions or alterations to sample types, stability, interfering substances, linearity, reference ranges, specificity and sensitivity
- Manufacturer’s telephone number
- Updates to supporting references
Labeling changes
You can expect to see changes to our product labeling.
For vials, tubes and cartridges, you may see:
- Addition of IFU symbol and web address for IFUs
- Addition of phone number under BEC address
- Addition of target fill volume
- Removal of approximate concentration from vial labels
- Removal of hazard codes
For product boxes, you will see the addition of the IVDR-required notified body number next to the CE mark.
Beckman Coulter Diagnostics products that will be covered under IVDR
-

Clinical Chemistry Systems
Reducing Total Cost of Ownership
In today's changing healthcare climate, laboratories around the world are challenged to elevate patient care and reduce operating costs at the same time. The low cost of ownership of our clinical chemistry analyzers help laboratories achieve their goals and manage resources effectively without compromising quality.
Learn how
your laboratory can:• Discover scalable solutions for laboratories of all sizes
• Standardize processes
-

Immunoassay Systems
Scalability, Reliability and Simplicity
Extend the services of your core laboratory with our range of immunoassay analyzers and comprehensive portfolio of assays. Whether you run dozens or thousands of tests per day, you can rely on the same innovation and quality in our immunoassay instruments and systems that you know and trust from the rest of our total laboratory solution portfolio.
Learn how your laboratory can:
• Scale with consistency
• Count on high levels of reliability
• Simplify the complex maintenance
• Support improved patient care
-

Protein Chemistry Systems
Supporting Disease Diagnosis and Monitoring
Are you seeking solutions that optimize and increase efficiency to advance patient care? Our protein chemistry analyzers combine the high performance needed to deliver fast, accurate results with the flexibility to accommodate a broad spectrum of laboratory preferences.
Learn how your laboratory can:
• Test with confidence
• Choose the right option
• Manage a range of diseases
• Evolve with innovation
-

Hematology
Comprehensive, High-quality Hematology Solutions
Deliver precise, accurate results faster. Use our hematology analyzers to enhance your medical laboratory operations and improve patient care.
Learn how
your laboratory can:• Achieve accurate results quickly with automated repeat and reflex testing to reduce TAT
• Reduce manual work, automate processes and use data management tools for auto-validation
• Free up workspace
• Make analytical testing more economical
-

Urinalysis
Take the Subjectivity Out of Urinalysis
Our selection of urine chemistry and urine microscopy analyzers help you significantly reduce your urinalysis laboratory's need for manual microscopic review and sample handling, thereby diminishing subjectivity and increasing test efficiency.
-

Microbiology
Guide Critical Patient Decisions Using Proven Accuracy
Impact treatment decisions with accurate detection of emerging antimicrobial resistance, improved workflow efficiency, expert consultation and top-ranked support.* Our selection of microbiology panels, systems and analyzers can enhance your laboratory’s productivity today and into the future.
-

Blood Banking
Industry-leading throughput, high efficiency and reliable performance
Our immunohematology and blood group testing analyzers and reagents deliver accurate results, while streamlining workflow and easing maintenance. Learn how your lab can experience industry-leading throughput, high efficiency and reliable performance.
-

Clinical Laboratory Automation Systems
Delivering Solutions Designed to Relieve Health System Pressure
Expedite patient care and enhance laboratory efficiency with our automated clinical laboratory systems. Experience automated lab solutions that deliver excellence for today and expandability for tomorrow.
References:
*MD Buyline. User Satisfaction Trending for Beckman Coulter Microbiology Systems. October 1, 2016.
1. 6 Major Differences Between EU’s MDR/IVDR and MDD/IVDD. (2019, March 14). RegDesk. https://www.regdesk.co/major-differences-eu-mdr-mdd/
2. FAQS: IN VITRO DIAGNOSTIC MEDICAL DEVICE REGULATION (IVDR). (2021). TUV. https://www.tuvsud.com/en-us/industries/healthcare-and-medical-devices/medical-devices-and-ivd/medical-device-market-approval-and-certification/eu-in-vitro-diagnostic-medical-device-regulation/faqs-in-vitro-diagnostic-medical-device-regulation-ivdr
3. https://www.medtecheurope.org/new-medical-technology-regulations/transition-periods/ accessed May 10, 2022
4. https://www.medtecheurope.org/wp-content/uploads/2020/05/20200526_Impact_Changes_Int_Reg_invitrodiagnostics_IVDR.pdf
 English
English

